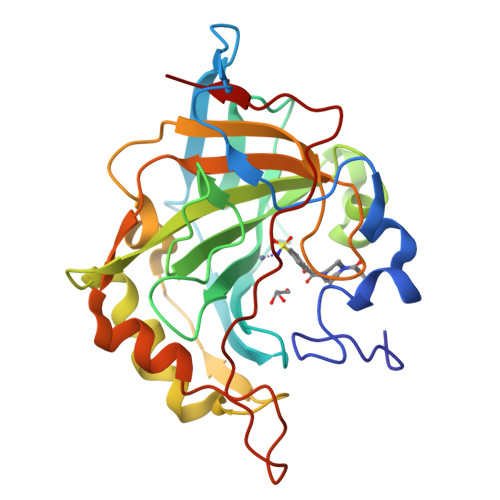
Looking toward the Rim of the Active Site Cavity of Druggable Human Carbonic Anhydrase Isoforms.
Mancuso, F., Di Fiore, A., De Luca, L., Angeli, A., Monti, S.M., De Simone, G., Supuran, C.T., Gitto, R.(2020) ACS Med Chem Lett 11: 1000-1005
- PubMed: 32435417
- DOI: https://doi.org/10.1021/acsmedchemlett.0c00062
- Primary Citation of Related Structures:
6XXT - PubMed Abstract:
We report the synthesis and biochemical evaluation of a series of substituted 4-(4-aroylpiperazine-1-carbonyl)benzenesulfonamides ( 5a - s ) developed as inhibitors of druggable carbonic anhydrase (CA) isoforms, as tools for the identification of new therapeutics. X-ray crystallography confirmed that this class of benzenesulfonamides binds CAs through the canonical anchoring of the benzenesulfonamide moiety to the metal ion and a tail-mediated recognition of the middle/top area of the active site cavity. Compound 5e (R = 2-Cl) demonstrated relevant selectivity toward brain-expressed hCA VII. The best balancing in binding affinity and selectivity toward tumor-expressed hCA IX/hCA XII over ubiquitous hCA I/hCA II was found for inhibitor 5o (R = 3-NO 2 ). Notably 5b (R = 2-F) proved to be the most efficacious inhibitor of hCA XII for which computational studies elucidated the CA recognition process.
Organizational Affiliation:
Dipartimento di Scienze Chimiche, Biologiche, Farmaceutiche ed Ambientali (CHIBIOFARAM), Università degli Studi di Messina, Viale Palatucci, Polo Didattico SS. Annunziata, 98168 Messina, Italy.